Within five years, the ability to track, trace and recall products throughout the supply chain will differentiate CPG companies that expand their business from those that close their doors. Executives who grasp the fundamentals of traceability will create environments in which change management and business practice reengineering seamlessly develop. Less promising scenarios await those who continue to operate in silos, regarding traceability as a collection of disparate technology fixes that satisfy ad hoc distributor requirements.
Wal-Mart mandates have made RFID the prominent traceability topic. Yet, RFID accounts for only one—albeit important—manifestation of good ERP systems. Successful technological applications stem from larger strategic frameworks. I ask a lot of executives, "What is your vision around tracking, tracing and recall?" Usually, they give precise answers. Next, I ask, "What are your objectives?" Here executives often falter, focusing on technology before developing a complete strategy.
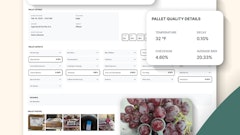
Fundamental traceability for non-durable goods starts with batch management, the ability to establish an identity for in-bound materials, materials produced or materials shipped. The batch concept allows manufacturers and distributors to track materials throughout production and distribution. Future RFID capabilities require an additional bedrock identity, called handling units, which allow tracking of pallets, cases and other containers.
Out of the blocks, you don't need RFID chips and RFID readers. This is a very common misconception. You need very basic components such as:
- Barcodes for all incoming materials;
- Ability to stage coded materials from warehouses to manufacturing facilities;
- Inventory and scanning capabilities;
- A system that integrates identities;
- Consuming goods to a line and re-scanning to ensure quality; and
- Ability to track materials outbound instructing distributors what to ship via EDI capabilities.
These building blocks clear the way for advanced applications, but also create the strategic foundation for change management and business process reengineering. The right ERP system makes every step of a product's life cycle recordable. From line operators to laboratory technicians, personnel records result at each stage of the supply chain. This functionality supports a key concept that brings integrity and accountability to each organizational role.
Line operators' physical inspections, combined with test results obtained by lab technicians, give a quality auditor the necessary information to reach a decision regarding disposition. These role concepts, available in a good CP solution, accommodate the segregation of duties. This segregation ensures supply chain integrity and prevents traceable connections from breaking down because a particular role underperformed.
This aspect is important as food companies begin to hold CEOs ultimately accountable for product safety. CEOs react to this charge by conveying the importance of individual responsibility to VPs of supply chain, quality management and manufacturing. Next, supervisors and directors receive the same message, which ultimately reaches the line operator. This chain messaging eventually results with individuals and unions requesting better training. Thus, executive accountability, operational accountability and employee accountability actualize change management, business process engineering and training practices.
Recent mass-scale food safety breaches resulted in hundreds of people getting sick from contaminated goods that reached restaurant and domestic tables. Such incidents will ultimately drive the requirements of HACCP and the Bioterrorism Act to the extent that the finished customer, whether a food service organization or individual customer, will force distributors and manufacturers to adopt preventative measures. The limited number of USDA and other agents cannot physically monitor all practices. Therefore, fulfillment of food laws depends on the integrity of manufacturers and distributors.
Currently, the pharmaceutical industry leads traceability efforts. The food industry is not there yet, but soon will be. As world population grows, production increases, creating bigger quantities of goods that demand freezing, chilling and storage. Each one of those steps presents increased risks. Unless companies manage those risks appropriately, by tracking and tracing, the risks will become unmanageable. In the event of a recall, you'll have to dispatch an army of vendors to every store and remove everything that's there, because you will not be able to determine what's good on the shelf and what's not good. Then, you'll have to replace the recalled goods, straining production, while customers vocally complain about a lack of inventory. The financial burden will be incredible.
Many executives remain skeptical of investing in traceability because the wrong solution can necessitate manual tracking and tracing. In these cases, personnel walk around lines and distribution centers with paper forms, recording compliance with passive requirements and Bioterrorism mandates. All this hard copy must be transferred to a database to generate the necessary reports. When visiting companies, I'm surprised by the cumbersome and haphazard processes in place. When executives discuss with me their business, within a few hours we find ways to dramatically reduce the number of steps. The executives come to understand how to encompass improved processes into their CP solution, letting the system do the work.
Change management is inevitable. Business process reengineering will become a concept embraced by CPG management and operators, within both manufacturing and distribution realms. The segregation of duties, propelled by traceability functionality, will ensure that all personnel recognize which batches to consume, mix and ship. This new culture of absolute interior responsibility will change business practices.