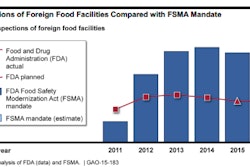
The Food Safety Modernization Act (FSMA), passed in 2011, mandates new systems for preventing produce contamination, and it requires more frequent inspections and controls for both domestic and foreign growers, shippers, importers, and distributors. In addition, it gives the FDA authority to stop or recall food products without gaining the input or approval of other agencies.
Following are four areas that anyone involved in the manufacture or distribution of food should focus on this year:
1. Document your food safety processes
This year, anyone involved in the food supply chain—including growers, manufacturers, and retailers—must document in an understandable, readable, and auditable way the procedures that your farm, plant, retail store, restaurant, or the like is taking to make sure that food does not get contaminated. Retailers will be pushing growers and shippers to provide this documentation. If a supplier does not have their processes documented, a retailer will likely refuse to accept product. Possibly, the FDA will decide to give smaller businesses more time to implement this phase, but the majority of the industry needs to address this challenge now.
2. Prepare to implement a track-and-trace system
Addressing the second phase of the new regulations, which applies to tracking and tracing food items through the supply chain, and maintaining associated records, is more complex.
A good model to follow is the Produce Traceability Initiative (PTI). This baseline 7-step track-and-trace program was implemented in 2012. Through this procedure of documenting one-up and one-down data on produce movements (recording where the product last came from and where it was subsequently sent to within the supply chain), growers, manufacturers, and retailers need to have documentation showing the product, lot number, and location of all food products so that they can be tracked and traced from their point of origin all the way through to point of sale or consumption. The FDA is still evolving its track-and-trace requirements, but I expect that they will model or at least overlap with PTI’s guidelines.
In the future, when the FDA extends its requirements to more food products, this means that retail stores must be able to show documentation by grower and by lot—not just by region—for every banana, grape, and any other food item it sells.
Currently, the FDA is not dictating whether the tracking and tracing should be accomplished through automation or manual recordkeeping. However, if you are not already using automated track and trace applications, like RFID or bar codes, now is the time to start exploring these systems and related technology.
3. Consider adoption of reusable containers
Reusable platforms are essential to track and trace for two important reasons. One, the use of standardized and uniquely identifiable and traceable reusable platforms enables the universal use of automated handling equipment and industry compliant scanning devices across all points in the supply chain. With permanently-embedded RFID tags in a container or platform, the unique tracking identifier (GRAI) can be easily read and recorded into tracking systems in a consistent and reliable way. Non-standard and non-tagged platforms do not allow for this level of automated tracking.
Two, the right reusable containers and washing systems can help meet the FDA’s call for improved sanitary practices including equipment, tool and container cleanliness. Reusable containers made of plastic and steel can minimize the potential for cross contamination, while certain washing systems and services can meet the most stringent of FDA requirements for sanitation. To learn more about the adoption of reusable products and services, visit the Reusable Packaging Association’s website at www.reusables.org.
4. Plan now for electronic data exchange in 2015
The ability to track and trace products and maintain product records and data for an extended period of time are the new reality under the FSMA. At the same time, the bigger challenge will be meeting the requirement for data to be easily exchanged among all parties in the supply chain now, or in 2015 when stronger levels of data recording conformance is predicted to be enforced.
Meeting this new regulation will require the industry to agree upon a standardized format—most likely GS1—for recordkeeping and the tracking and tracing of food products. The tracking and tracing of individual food items via a consistent method of labeling, documenting, and reporting that all can have access to is likely to become the norm, rather than the exception, under the new rules.