In the United States, lawmakers, regulators, consumers and the media are more focused on food safety than ever before. On January 4, 2011, President Obama signed the Food Safety Modernization Act into law, which represents the largest change of the food safety system since the passage for the Food, Drug, and Cosmetic Act in 1938. The new legislation will bring significant changes to an already shifting food safety landscape, especially in the area of imported foods and ingredients. This paper will outline (1) the current food safety landscape that has impact on the way in which food imports are perceived in the U.S., (2) an overview of the key provisions of the new legislation of importance to importers and exporters, (3) additional considerations, and opportunities.
Food Safety Landscape
Changing consumption patterns, large outbreaks of foodborne illness, new food safety science, and identification of new risks all contribute to the shifting food safety system. Consumer demand for fresh food year-round requires global sourcing of fresh food and ingredients. Currently, imports account for 15 percent of the U.S. food supply, including over 75 percent of seafood and 60 percent of fresh produce consumed in the U.S.
Numerous outbreaks over the past several years have made media, consumers, and the United States Congress keep a closer watch on food safety systems.
In response to the changing food safety landscape, United States regulatory agencies are cracking down on food safety violations which will result in faster, more frequent enforcement actions.
The expanding global food supply has resulted in an increased regulatory presence overseas, at the border, and domestically. FDA has opened foreign offices in China, India, Latin America, Europe, and the Middle East (currently based out of Washington, D.C.). FDA is implementing a predictive risk-based screening system at the border to better target field exams and speed up the importation of low-risk products. FDA is increasing inspections, both in the U.S. and overseas, and is leveraging with federal, state, local, tribal, and territorial partners to provide oversight of the U.S. food system.
Food Safety Modernization Act: Key Provisions
The Food Safety Modernization Act (FSMA) contains several provisions that give FDA new authority and place new requirements on those involved in the supply chain.
The primary aim of FSMA is to require food companies to implement preventive controls. In order to do that companies need to understand the risk both in their facility and in their supply chain and develop control systems to control that risk using validated systems, and document that those controls are working and maintain the records showing that the controls are working. One of the most important sections of FSMA is Section 103 that focuses on these preventive controls. Understanding this section is critical to meet the requirements that will be enacted with regard to imported foods.
Sec 103. Preventive Controls
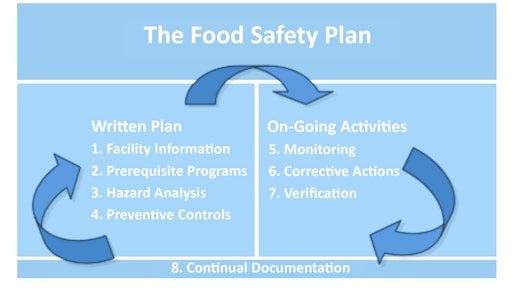
One of the most anticipated sections of FSMA for all food manufacturers – whether inside or outside the United States – relates to the new requirement that all facilities that manufacture, process, pack or hold food products have documented preventive control programs in place. The preventive control requirements will go above and beyond a typical Hazard Analysis and Critical Control Point plan, and will include requirements related to recall plans, sanitation, employee hygiene training, environmental monitoring, allergen control program, current good manufacturing practices, supplier approval and verification activities, corrective actions, verification activities, and records. As we move beyond HACCP, preventative control programs should include training programs for managers and/or workers, audit programs, written records (e.g., batch records, sanitation records, etc.), validation of control measures, written sanitation standard operating procedures, food label review and control program, and testing of in-coming raw materials, in-process materials, finished products, and processing environment. Monitoring requirements must verify that controls are working correctly and are effectively and significantly minimizing or preventing the occurrence of identified hazards. Food safety plans will need to be developed, (see food safety plan resource box, next page).
This is one of the key rules that was expected to be released in January 2012 but has been delayed. All members of the food supply chain should pay close attention to these requirements and related guidance documents as soon as they become available.
Sec 105. Standards for Produce Safety
For the first time, FDA is requiring those who produce fruits and vegetables determined to be “high risk” based on their association with outbreaks to enact measures to ensure the safe production and harvesting of the produce. These requirements will apply to both domestic and imported produce and the measures include controls over soil amendments, hygiene, packaging, temperature controls, animals, and water, so as to reduce or eliminate the likelihood of both natural hazards as well as those that may be intentionally introduced from impacting the safety of the product. Like the preventive control rule, stakeholders are anxiously awaiting the release of this rule.
Sec. 301 Foreign Supplier Verification Program
FSMA is moving the burden of imported food safety onto importers. This section of FSMA lays out how importers are going to be held more accountable for the safety of food products and ingredients they import into the U.S. Importers will need to perform risk-based foreign supplier verifications activities to ensure that food entering the US has been produced by firms employing preventive controls (as described in Sec. 103) unless the food is already required to be manufactured using HACCP-based preventative controls (seafood, juice, low acid canned products).
This program, scheduled to be implemented in January 2013 but likely to be delayed, is dependent on the issuance of the preventive control rules that are currently delayed. This section of FSMA is significant for importers of foods and ingredients, as it makes them responsible for ensuring that the products they handle are safe. Thus, supply chain visibility will be crucial for compliance.
Sec. 302 Voluntary Qualified Importer Program (“Green Lane”)
FDA is required to establish a voluntary program that would provide expedited review of food and ingredients from participating importers. The program will be paid for by program participants (as specified in Section 207). Eligibility to participate in the program will depend, in part, on the known safety risk of the food product or ingredient to be imported; the compliance history of foreign suppliers used by an importer; the capability of the regulatory system of the country of export to ensure compliance with U.S. food-safety standards; recordkeeping, testing, inspection, and audits of facilities; traceability throughout the supply chain; proof of consistent temperature controls; and the potential risk for intentional adulteration of the food. The voluntary program will require the issuance of a facility certification to accompany products brought into the U.S. under this program.
Details emanating from FDA regarding this program have been substantially delayed, and it will likely be years until the program is fully implemented. However, for those exporting to the US, this should be an area of key interest.
In addition to the key provisions described above, there are a number of provisions that impact importers. Additional information can be found at http://leavittpartners.com/global-food-solutions/.
Sec. 104 Performance Standards
After periodic review of foodborne contaminant health data, including toxicological and epidemiological studies, FDA may issue guidance documents regarding action levels or regulations based on recommendations of advisory committees, including the Food Advisory Committee.
Sec. 115 Port Shopping
FDA has to provide notification to the Secretary of Homeland Security when refusing to admit food so that other US ports are aware that the food has previously been refused entry.
Sec. 303 Authority to Require Import Certifications for Food
Although unlikely to be required immediately, FDA now has the authority to require certificates issued by independent third parties or governments verifying that the food is in compliance with U.S. laws and regulations. FDA will use a risk-based approach to determine foods and ingredients for which a certificate will be required.
Sec. 306 Inspection of Foreign Food Facilities
The Secretary may enter into arrangements and agreements with foreign governments to fulfill FDA’s mandate to conduct the 19,000 annual inspections required by 2016. Inspections will be targeted based on risk.
Sec. 307 Accreditation of Third-Party Auditors
The legislation requires FDA to establish a program to recognize accreditation bodies and third-party auditors including foreign governments, foreign cooperatives or a private entity. This audit will determine if a facility is eligible to offer food for import under the voluntary qualified importer program.
Food Safety Modernization Act: Additional Considerations & Opportunities
Multiple drivers are currently impacting the food safety landscape and many changes are in motion, independent of the new legislation. The implementation of the new legislation will take time and is largely dependent on funding and how FDA drafts the regulations. The extent to which the U.S. Congress funds food safety will directly impact how much FDA can accomplish towards increasing inspections, and implementing new programs. However, the FDA will proceed with the writing of the regulations and thus set expectations and requirements.
The new legislation won’t change the food safety landscape overnight, but there is a unique opportunity for industry leaders to share best-practices with FDA, participate in shaping the new regulations and guidance, and position themselves to have the solutions to meet the needs created by the new requirements.
By following the developments in U.S. regulatory policy over the coming years and evaluating your business practices against current standards, when the time comes for FDA to open a new “Green Lane,” your company could be one of the first to gain expedited clearance for your products into the United States. Lining-up and working with leading independent third party auditors consultants, laboratories or foreign governments to accomplish this goal is critical to providing the necessary assurances to FDA that a protective and reliable food safety system is in place for your imported products.
This paper was co-authored by Leavitt Partners and Eurofins. For more information, contact James Acheson at [email protected]